Magnesium Hydroxide Ksp Expression . 176 rows the solubility product is given by. The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. First, write out the k sp expression, then substitute in concentrations and solve for k sp: The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and its respective. For every mole of magnesium hydroxide that dissolves, you. Write the chemical equation showing how the substance. Writing [hg +] 2 in the k sp expression is wrong. From the balanced dissolution equilibrium, determine the equilibrium concentrations of the dissolved solute ions. Here are ten chemical formulas. 176 rows they are calculated by using standard state thermodynamic data and the equations: The value of the solubility constant depends only on. Calculate the solubility of magnesium hydroxide in grams per.
from www.chegg.com
The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. From the balanced dissolution equilibrium, determine the equilibrium concentrations of the dissolved solute ions. 176 rows they are calculated by using standard state thermodynamic data and the equations: Here are ten chemical formulas. Writing [hg +] 2 in the k sp expression is wrong. For every mole of magnesium hydroxide that dissolves, you. Calculate the solubility of magnesium hydroxide in grams per. Write the chemical equation showing how the substance. 176 rows the solubility product is given by. The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and its respective.
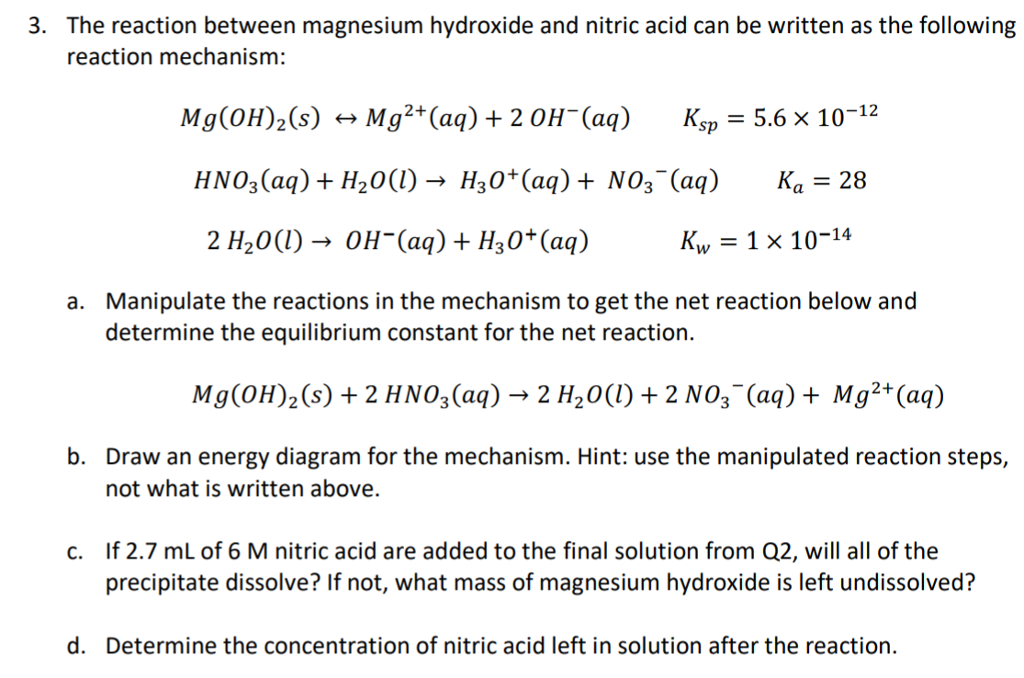
3. The reaction between magnesium hydroxide and
Magnesium Hydroxide Ksp Expression The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and its respective. For every mole of magnesium hydroxide that dissolves, you. From the balanced dissolution equilibrium, determine the equilibrium concentrations of the dissolved solute ions. Calculate the solubility of magnesium hydroxide in grams per. Here are ten chemical formulas. 176 rows the solubility product is given by. The value of the solubility constant depends only on. The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. Writing [hg +] 2 in the k sp expression is wrong. First, write out the k sp expression, then substitute in concentrations and solve for k sp: 176 rows they are calculated by using standard state thermodynamic data and the equations: Write the chemical equation showing how the substance.
From www.chegg.com
Solved PostLaboratory Question The Ksp of magnesium Magnesium Hydroxide Ksp Expression Here are ten chemical formulas. For every mole of magnesium hydroxide that dissolves, you. Writing [hg +] 2 in the k sp expression is wrong. The value of the solubility constant depends only on. Write the chemical equation showing how the substance. The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. The solubility. Magnesium Hydroxide Ksp Expression.
From www.researchgate.net
(PDF) Magnesium Hydroxide Nanoparticles Inhibit the Biofilm Formation Magnesium Hydroxide Ksp Expression 176 rows they are calculated by using standard state thermodynamic data and the equations: Write the chemical equation showing how the substance. For every mole of magnesium hydroxide that dissolves, you. Writing [hg +] 2 in the k sp expression is wrong. The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between. Magnesium Hydroxide Ksp Expression.
From www.mdpi.com
Processes Free FullText Solubility Data of Potential Salts in the Magnesium Hydroxide Ksp Expression Calculate the solubility of magnesium hydroxide in grams per. The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. Here are ten chemical formulas. Writing [hg +] 2 in the k sp expression is wrong. 176 rows the solubility product is given by. The value of the solubility constant depends only on. From the. Magnesium Hydroxide Ksp Expression.
From www.numerade.com
SOLVED A. Calculate the solubility (in g/L) of magnesium fluoride (Ksp Magnesium Hydroxide Ksp Expression 176 rows they are calculated by using standard state thermodynamic data and the equations: First, write out the k sp expression, then substitute in concentrations and solve for k sp: Calculate the solubility of magnesium hydroxide in grams per. Writing [hg +] 2 in the k sp expression is wrong. The value of the solubility constant depends only on. From. Magnesium Hydroxide Ksp Expression.
From www.aiophotoz.com
How To Find Molar Solubility Given Ksp Images and Photos finder Magnesium Hydroxide Ksp Expression From the balanced dissolution equilibrium, determine the equilibrium concentrations of the dissolved solute ions. For every mole of magnesium hydroxide that dissolves, you. The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and. Magnesium Hydroxide Ksp Expression.
From www.metabolics.com
Magnesium Hydroxide Metabolics Magnesium Hydroxide Ksp Expression For every mole of magnesium hydroxide that dissolves, you. Here are ten chemical formulas. The value of the solubility constant depends only on. Calculate the solubility of magnesium hydroxide in grams per. Writing [hg +] 2 in the k sp expression is wrong. Write the chemical equation showing how the substance. 176 rows they are calculated by using standard state. Magnesium Hydroxide Ksp Expression.
From www.numerade.com
SOLVED Question 5 What is the equilibrium constant expression for the Magnesium Hydroxide Ksp Expression The value of the solubility constant depends only on. 176 rows the solubility product is given by. The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and its respective. Write the chemical equation showing how the substance. For every mole of magnesium hydroxide that dissolves, you. Writing [hg +]. Magnesium Hydroxide Ksp Expression.
From prescriptiongiant.com
Magnesium Hydroxide Prescriptiongiant Magnesium Hydroxide Ksp Expression 176 rows the solubility product is given by. Writing [hg +] 2 in the k sp expression is wrong. For every mole of magnesium hydroxide that dissolves, you. First, write out the k sp expression, then substitute in concentrations and solve for k sp: The value of the solubility constant depends only on. Here are ten chemical formulas. 176 rows. Magnesium Hydroxide Ksp Expression.
From www.researchgate.net
Contact Angle of magnesium hydroxide before and after modification Magnesium Hydroxide Ksp Expression Writing [hg +] 2 in the k sp expression is wrong. First, write out the k sp expression, then substitute in concentrations and solve for k sp: 176 rows the solubility product is given by. The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and its respective. The solubility. Magnesium Hydroxide Ksp Expression.
From www.chegg.com
3. The reaction between magnesium hydroxide and Magnesium Hydroxide Ksp Expression First, write out the k sp expression, then substitute in concentrations and solve for k sp: Calculate the solubility of magnesium hydroxide in grams per. Here are ten chemical formulas. 176 rows they are calculated by using standard state thermodynamic data and the equations: The value of the solubility constant depends only on. From the balanced dissolution equilibrium, determine the. Magnesium Hydroxide Ksp Expression.
From study.com
Writing a Solubility Product (Ksp) Expression Chemistry Magnesium Hydroxide Ksp Expression For every mole of magnesium hydroxide that dissolves, you. Writing [hg +] 2 in the k sp expression is wrong. First, write out the k sp expression, then substitute in concentrations and solve for k sp: Write the chemical equation showing how the substance. The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11.. Magnesium Hydroxide Ksp Expression.
From eureka.patsnap.com
magnesium hydroxide composite material as well as preparation Magnesium Hydroxide Ksp Expression 176 rows the solubility product is given by. Writing [hg +] 2 in the k sp expression is wrong. Calculate the solubility of magnesium hydroxide in grams per. The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. For every mole of magnesium hydroxide that dissolves, you. First, write out the k sp expression,. Magnesium Hydroxide Ksp Expression.
From www.numerade.com
SOLVED 14. Use the information below to answer this question. A Magnesium Hydroxide Ksp Expression For every mole of magnesium hydroxide that dissolves, you. The value of the solubility constant depends only on. The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and its respective. Writing [hg +]. Magnesium Hydroxide Ksp Expression.
From askfilo.com
The pH of aqueous solution of magnesium hydroxide is 9.0. If Ksp of magn.. Magnesium Hydroxide Ksp Expression For every mole of magnesium hydroxide that dissolves, you. Here are ten chemical formulas. 176 rows they are calculated by using standard state thermodynamic data and the equations: First, write out the k sp expression, then substitute in concentrations and solve for k sp: The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11.. Magnesium Hydroxide Ksp Expression.
From www.chegg.com
Solved The Molar Solubility Of Magnesium Hydroxide Is 1.1... Magnesium Hydroxide Ksp Expression From the balanced dissolution equilibrium, determine the equilibrium concentrations of the dissolved solute ions. Write the chemical equation showing how the substance. Writing [hg +] 2 in the k sp expression is wrong. First, write out the k sp expression, then substitute in concentrations and solve for k sp: The value of the solubility constant depends only on. Here are. Magnesium Hydroxide Ksp Expression.
From www.numerade.com
SOLVEDFor the dissolution of magnesium hydroxide in water (a) Write a Magnesium Hydroxide Ksp Expression First, write out the k sp expression, then substitute in concentrations and solve for k sp: The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and its respective. Calculate the solubility of magnesium hydroxide in grams per. Write the chemical equation showing how the substance. 176 rows the solubility. Magnesium Hydroxide Ksp Expression.
From www.youtube.com
Find the Ksp of magnesium phosphate YouTube Magnesium Hydroxide Ksp Expression The solubility product constant, often denoted as k sp, is a numerical value that represents the equilibrium between a solid and its respective. 176 rows the solubility product is given by. Write the chemical equation showing how the substance. From the balanced dissolution equilibrium, determine the equilibrium concentrations of the dissolved solute ions. First, write out the k sp expression,. Magnesium Hydroxide Ksp Expression.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn by Sri Chaitanya Magnesium Hydroxide Ksp Expression The solubility product of magnesium hydroxide mg(oh) 2 at 25° c is 1.4 × 10 −11. Writing [hg +] 2 in the k sp expression is wrong. From the balanced dissolution equilibrium, determine the equilibrium concentrations of the dissolved solute ions. Here are ten chemical formulas. The solubility product constant, often denoted as k sp, is a numerical value that. Magnesium Hydroxide Ksp Expression.